Research Announcements
Below is information about research opportunities for your patients with inborn errors of metabolism. If you would like to post about research in which you are conducting, please contact the GMDI Research Committee.
Stay up to date on what research opportunities are available for your patients.
Posted 3/5/26

Online Survey Study in LC-FAOD Recruiting Participants in the US and Canada
Ultragenyx is conducting an online survey study in Long Chain Fatty Acid Disorders (LC-FAODs).
This survey is being conducted to better understand current treatment practices across all 6 LC-FAOD types, including variations based on patient age and disease severity. Survey responses will be analyzed and reported in aggregate. No individual-level identifying information will be included in any reporting. Personal contact information, if collected, will be solely for survey administration and compensation purposes.
If you are interested in completing the survey, please contact Amie Thompson at aithompson@ultragenyx.com for the survey link and further information.
Eligible participants who complete the 45-minute survey will receive fair market value compensation for their time, not contingent upon survey responses.
Thank you for your consideration!
Posted 7/24/25
flok Health is pleased to announce the launch of flok Research, an IRB-approved platform designed to advance research in inherited metabolic disorders (IMDs)—powered by the lived experiences of people managing these conditions every day. Details at https://research.flok.org
Through flok Research, individuals with PKU, MSUD, Classical Homocystinuria, Tyrosinemia, Organic Acidemias, and UCDs can contribute to research simply by using the flok app—where daily care data becomes research insights.
Details below – please share the attached materials with colleagues and patients who may be interested.
Current studies:
- flok Research, our longitudinal study designed to better understand the lived experience of those with inherited disorders of protein metabolism, characterize how these conditions and their treatments impact health, and improve care by accelerating scientific research. This study is open to all ages and has no fixed end date. Details here: https://flok.link/gmdi-lt [Download study flyer]
- The Signal Study, a 12-week cohort study for adults with inherited disorders of protein metabolism. This study will explore how use of the flok app and an Oura ring can capture meaningful signals—like diet, sleep, symptoms, and wellbeing—in real time. https://flok.link/gmdi-sgnl [Download study flyer]
Users may consent in-app: Main Menu > Manage Consent, after which they are eligible to apply to join the Signal Study.
Questions can be directed to support@flok.org
https://research.flok.org
Sponsor: flok Health: (https://flok.org)
Both studies are covered under North Star IRB #NB500298.
Posted 7/21/25
UCLA Division of Clinical Genetics and Division of Pediatric Allergy, Immunology, and Rheumatology collaborated in 2023 to develop "The Metabolic Dietitian's Guide to Introducing Food Allergens for Babies with Amino Acid Disorders" and disorder-specific supporting patient-education materials. The impetus for this was based in a shift in our understanding of food allergies and how they develop, which has led to a drastic change in how we introduce potential food allergens to infants.
These clinical practice tools and patient education materials for introducing food allergens in infants with amino acid disorders have been circulated and utilized by Metabolic Clinics around the world since late 2023/early 2024. At this point, it is important to evaluate the applicability and effectiveness of the clinical practice tools and patient education materials that have been developed.
You are invited to complete a survey (IRB-25-1102). The feedback we receive from this survey will help inform how these tools are being utilized and ways in which they can be optimized to continue to improve patient care. Feedback is captured anonymously. We estimate that the survey will take 5-8 minutes to complete.
Survey link: https://form.jotform.com/251905726178060
Deadline date: September 1, 2025
Chanel Suares, RD, Senior Metabolic Dietitian, UCLA Division of Clinical Genetics
Sarah Roberts, MS, RD, Metabolic Dietitian, University of Utah Division of Medical Genetics
Catherine Manalo, RD, CNSC, Clinical Dietitian IV/Metabolic Dietitian, CHOC Division of Metabolic Disorders
Posted 6/24/25

We are recruiting participants to help validate a nutrition application called MyRareDiet. Eligible participants include those with a diagnosis of a UCD, MUSD, PA and MMA. Study information and how to contact someone about participating are pasted below. If you have eligible patients who might be interested, please share this information with them. Participants will receive $200 for completing the fully remote study.
If you are interested in MyRareDiet, you can find the news article we published recently here: Overview of MyRareDiet™: A diet tracking tool for patients with inborn errors of metabolism.
Interested participants can provide their information here: interest form link: https://nucdf.org/mrd-contact/myraredietparticipation.html.
Thanks so much for telling potential participants about this opportunity.
Melanie Gillingham, PhD, RD
Professor
Molecular and Medical Genetics
Graduate Programs in Human Nutrition
Oregon Health & Science University
Email: gillingm@ohsu.edu
Posted 6/5/25

Uncommon Cures is conducting a Phase 4 study for PKU patients experiencing hypersensitivity reactions to Palynziq (pegvaliase) on behalf of BioMarin Pharmaceutical Inc. The study is a phase 4 study to evaluate the impact of rapid drug desensitization (RDD) protocol for adults with phenylketonuria experiencing hypersensitivity reactions to Palynziq.
The study is up to 30 weeks long. There is a 42-day screening period (remote), 2days of visits at the study center which include one full day of RDD and a second day of dosing of Palynziq in the study center (about 2 hours), followed by 24 weeks of remote follow up after the RDD. Following RDD, patients will resume their Palynziq at the dose at which their qualifying hypersensitivity reaction initially occurred. Patients will have immunogenicity lab assessments on day 1, day 2,and via mobile phlebotomy during week 24. All travel and lodging expenses are covered by the study.
Participants must be 18 years of age or older with phenylketonuria, be prescribed Palynziq, have developed hypersensitivity reactions that have led to interruption of treatment or reduction of dose or dosing frequency, and must be able to undergo RDD within 6 weeks from the hypersensitivity reaction. Please let us know if you would like to discuss this study further or if you have any patients who may be eligible, and we can provide additional information.
For more information please contact:
Posted May 26, 2026
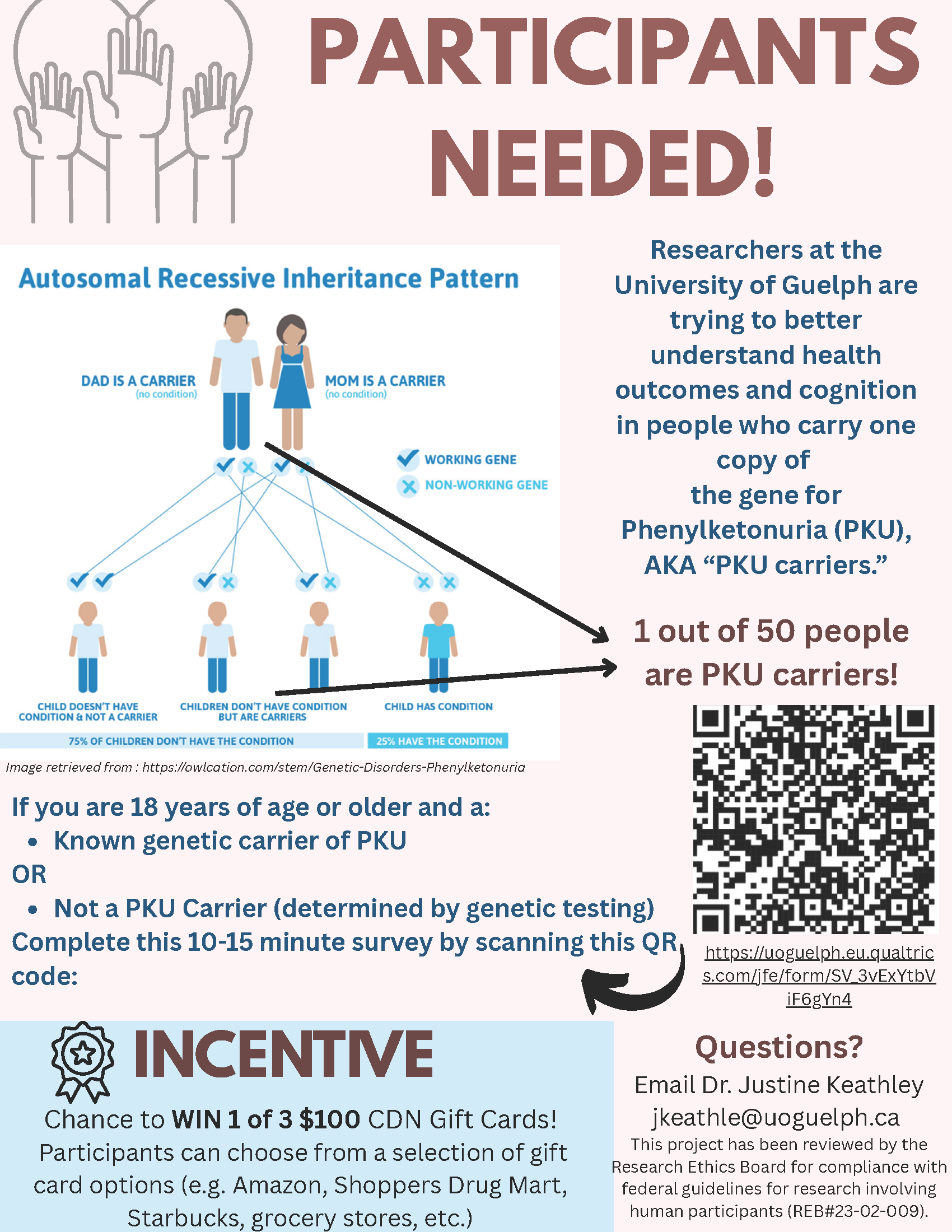
Posted 5/2/25


Posted 3/14/25
We invite you to contribute to an important initiative aimed at enhancing the care and support for women with phenylketonuria (PKU) experiencing perimenopausal and menopausal symptoms.
Menopause marks a significant transition in a woman's life, typically confirmed after 12 consecutive months without a menstrual period. The preceding phase, known as perimenopause, is characterised by hormonal fluctuations and various symptoms, including hot flushes, sleep disturbances, and mood changes.
At the Royal Melbourne Hospital (RMH), we have identified notable challenges faced by women with PKU during this critical period. These challenges include rising phenylalanine (phe) levels, weight gain, and mental health difficulties, which can significantly impact their overall quality of life and engagement in care.
To better understand these issues, we are conducting a survey targeting women aged 45-55 years with PKU, diagnosed through newborn screening, who are currently experiencing perimenopausal or menopausal symptoms.
Your participation is invaluable to enabling us to better understand this patient cohort and ultimately support them better too! By participating, you will help us gather valuable insights that can lead to improved care strategies and support systems for this vulnerable population. Once the results are compiled, we will share the findings with all participants, fostering a collaborative approach to addressing these challenges.
This survey will take approximately 20-30 minutes to complete. Please complete this survey by clicking on the link below by Wednesday 30 th April to enable data collation and initial results.
PKU and Menopause
Thank you for considering this opportunity to make a difference in the lives of women with PKU. Your expertise and insights are invaluable to us.
Claire Rutledge
Metabolic Dietitian
Metabolic Diseases Unit
Royal Melbourne Hospital
Claire.rutledge@mh.org.au
Ph: 9342 7074
Fax: 9342 4808
Posted 2/25/25

Posted 2/24/25


Posted January 9, 2025
Online Survey Study in MCADD Recruiting Participants in the US and Canada
Ultragenyx, in partnership with Lumanity, is conducting an online survey study in Medium-Chain Acyl-Coenzyme A Dehydrogenase Deficiency (MCADD).
This survey study is being conducted to better understand the experiences of adult patients living with MCADD and caregivers of pediatric patients living with MCADD. Eligible participants will be paid $50 for completion of the online survey.
Do you treat or manage adult patients and/or caregivers of pediatric patients with MCADD who could be eligible for this study (please see below for inclusion/exclusion criteria)? If so, you can help with recruitment by:
- Informing adult patients and/or caregivers of pediatric patients with MCADD about this study; and
- Sharing the study link or QR code provided below with interested adult patients and/or caregivers of pediatric patients with MCADD
Who may qualify:
- Patients located in the US and Canada
- Adult patients (i.e. over the age of majority where they live) with a confirmed diagnosis of MCADD or legal guardians/primary caregivers of a pediatric patient (i.e. younger than the age of majority where they live) living with MCADD
- Participants able to read, write, and comfortably communicate in English
- Participants willing and able to participate in a 30-minute online survey
- Participants able to describe age the formal MCADD diagnosis was made
- Participants able to describe what event(s) led to a formal MCADD diagnosis
- Participants able to describe the speciality of the physician who provided a formal MCADD diagnosis
- If needed, participants able to participate in a phone screening to answer additional questions regarding their diagnostic journey or the diagnostic journey of the person living with MCADD
Who may not qualify
- Participants unable to understand the nature, scope, and consequences of the survey study and presents evidence of an uncooperative attitude
- Caregiver participant is paid to provide care for the person living with MCADD
We would appreciate if you would tell your patients and their caregivers to reach out to mcadd@lumanity.com if they are interested in participating.
Interested patients and caregivers can also scan the QR code below:

Download PDF Flyer
Posted December 17, 2024
The survey will remain open until January 3, 2025
Posted October 7, 2024
The OTC-HOPE study is a Phase 1/2 first-in-human clinical trial of ECUR-506 in newborn males with genetically confirmed neonatal onset OTC deficiency and will test differing dose levels of ECUR-506. The study is enrolling newborn males up to seven months of age who are diagnosed with severe neonatal onset OTC deficiency and meet certain other criteria. The primary objective is to assess the safety and tolerability of intravenous administration of a single dose of ECUR-506. It will also assess the pharmacokinetics and efficacy of ECUR-506 administration and the potential effects of ECUR-506 on disease-specific biologic markers, developmental milestones and quality of life.
iECURE’s approach to gene editing for its initial programs, including OTC deficiency, relies on the delivery of two adeno-associated virus (AAV) capsids, each carrying different payloads. ECUR-506 comprises two vectors, an ARCUS® nuclease vector targeting gene editing in the well-characterized PCSK9 gene locus and a donor vector that inserts the desired functional OTC gene. iECURE has licensed the ARCUS nuclease for ECUR-506 from Precision BioSciences. The cut in the PCSK9 site serves as the insertion site for the OTC gene, providing a potential path to permanent expression of a healthy gene. ECUR-506 is being studied in the OTC-HOPE study, the first clinical meganuclease-based in vivo gene insertion program. The ECUR-506 program is the first in vivo gene insertion program to be cleared in the U.S. for study in infants, and it represents the first time that the ARCUS® nuclease has been used to provide an in vivo insertion point for a functional gene in the clinic.
Click HERE to download flyer
For more information, visit https://otc-hope.com/
For additional questions, please reach out to medinfo@iecure.com
Posted July 11, 2024
Dear Esteemed Colleagues,
We are excited to announce that our rare disease clinical trial center, Uncommon Cures (uncommoncures.com), is currently enrolling participants for the ARCT-810-04 study. This innovative Phase 2a trial is exploring the potential of ARCT-810, an investigational mRNA therapy designed to address the root cause of Ornithine Transcarbamylase (OTC) deficiency.
ARCT-810 utilizes mRNA as a template for producing the OTC enzyme in the liver, leveraging Arcturus's proprietary LUNAR® lipid nanoparticle technology for effective delivery. This open-label, multiple ascending dose study aims to evaluate the drug's pharmacodynamics, safety, and preliminary efficacy in restoring urea cycle function.
Eligibility Criteria:
- Adolescents and adults aged 12 and older
- Confirmed diagnosis of OTC deficiency
- Medical history of ammonia or glutamine elevations
- Currently on stable therapy and/or dietary interventions for OTC deficiency
Study Details:
- No placebo group involved
- Participants will receive 5 intravenous infusions of ARCT-810 every two weeks, at one of three dose levels
- Approximately 13 visits over a 16-week period, including a screening visit
Location: Uncommon Cures, Chevy Chase, Maryland
All study-related laboratory tests, medical exams, and travel are provided at no cost.
For any inquiries, please feel free to contact us at Arcturus@uncommoncures.com or Community@ArcturusRx.com.
Thank you.
Tamanna Roshan Lal, MB ChB
Sub-Investigator
Uncommon Cures
Chevy Chase, MD
Informational Flyer
Clinical Trial Flyer
Posted January 22, 2024

Study now recruiting for adults living with PKU, with option to participate fully remotely!
Synlogic is conducting a novel research study with an orally administered medication titled: Efficacy and Safety of SYNB1934 in Patients with PKU (SYNPHENY-3).
This is a double blind, randomized, placebo control Phase-3 trial, and we are seeking participants. Our investigational medication labafenogene marselecobac (SYNB1934) is derived from the probiotic Escherichia coli Nissle 1917 (EcN) in a series of genetic manipulations. It is designed to allow enhanced degradation of Phe within the human gut before it enters the main circulation.
The Sympheny-3 study is currently recruiting participants. This is the first IEM trial offering a fully virtual option for patients, in addition to physical trial sites to increase diversity and accessibility. The study is being run by board-certified physicians and has been approved by the Advarra Institutional Review Board.
We are seeking your help in recruiting patients with PKU! We appreciate if you would tell your patients to visit: www.pkuresearchstudy.com.
Who may qualify:
- Adults 18 years and older at the time of consent
- Diagnosed with PKU and unable to maintain blood Phe levels below 360 micromol/L.
- Demonstrate an average Phe level >360 micromol/L from three blood samples prior to enrollment
- Patients taking concurrent PKU medications such as LNAA, Kuvan/sapropterin or sepiapterin are permissible and welcomed.
- Patients must remain stable on their dietary prescription during parts of the trial.
For more information on the study inclusion criteria, as well as contact information for the virtual or traditional study sites, visit ClinicalTrials.gov, study ID NCT05764239.
If you have questions, please reach out to Angela Kurtz, M.S., RD, CDN (angela.kurtz@synlogictx.com). We appreciate your support and interest in this study!
This above information is intended for HCPs only.
Please share the study link with interested patients.
______________________________________________________________________________________________________________
Posted December 6, 2023
Laura Sliwoski is a metabolic dietitian with Oregon Health & Science University in Portland, Oregon. She is studying how the national formula shortage affected metabolic dietitians and their patients with inborn errors of metabolism (IEM).
You are invited to participate in an online survey where you will answer questions about your experience during the formula shortage, including the added burden and stress of taking on additional work, the nutritional impacts on patients, and the strain on the patient-clinician relationship.
Dietitians who work with patients with an IEM within the United States are eligible to participate. The survey will take about 15 minutes to complete. As a thank you for your time and participation, you have the option to be entered in a raffle to win a $100 Visa gift card.
To learn more about the study, scan the QR code below or visit https://redcap.link/ayxtqo6j. More Info

Additionally, there is a second survey to understand how the formula shortage affected patients with an IEM and their families. Patients with an IEM who are 18 years and older are eligible, as well as parents/caregivers of a child with an IEM who is less than 18 years of age. Parents/caregivers of an adult with an IEM who is decisionally impaired are also eligible to participate. The patient and parent/caregiver survey are available in English and Spanish. All participants will have the option to be entered in a raffle to win a $100 Visa gift card.
To share the patient and parent/caregiver survey with your patients, please have them scan the QR code below or visit https://redcap.link/6od39e6q. More Info

For questions, or to speak with the study team, please call 503-494-3137. We appreciate your interest in this study!
______________________________________________________________________________________________________________
Posted October 18, 2023
The Midwest Genetics Network (MGN) is conducting a research project survey entitled “Assessing provider perspectives, needs and barriers to implementing trauma-informed care in medical clinics serving patients with genetic conditions” to assess trauma-informed care in medical clinics. We are looking for clinicians and healthcare providers who regularly provide care to individuals with diagnosed or suspected genetic conditions. This may include (but is not limited to) physicians, advanced practice providers (NPs, PAs), genetic counselors, and registered dieticians. The purpose of the survey is to assess the perspectives and experiences of genetic providers on trauma-informed care and any barriers to implementing trauma-informed protocols within their clinics. We greatly appreciate your participation in this survey. The information you provide will be used to inform the development of resources and guidance for implementing trauma-informed protocols and guidelines in genetics clinics in order to improve care for patients with genetic conditions.
Your individual responses are confidential. The information gathered will be published in an aggregated summary. Genetic providers will receive a $10 Amazon e-gift card for participation. The survey will take about 15-20 minutes of your time.
Additionally, you will be asked if you are interested in participating in a research project focus group to further discuss your experiences with trauma-informed care. A form at the end of the survey will be provided to enter your contact information to receive an additional $50 Amazon e-gift card and follow-up in the study. Information provided in the form will not be tied to your survey response. Please use this link to complete the survey:
Any questions can be directed to Kelsey Sala-Hamrick at ksalaham@mphi.org
The survey will remain open until February, 2024.
Study Flyer
______________________________________________________________________________________________________________
Posted September 18, 2023
Validation of the Phenylketonuria Symptom Severity and Impacts Scale (PKU-SSIS) v2.0 in Adolescents with PKU

BioMarin Pharmaceutical Inc. developed the Phenylketonuria Symptom Severity and Impacts Scale (PKU-SSIS), a self-administered disease-specific measure to understand the experience of people with PKU and its impacts on their everyday life. The PKU-SSIS Version 2.0 was recently adapted for adolescents (ages 12 to 17 years, inclusive) with PKU. This study aims to conduct online surveys with 75 to 100 adolescents with PKU and their parents/guardians at two timepoints (10 to 14 days between timepoints) to validate the PKU-SSIS Version 2.0 for use in the adolescent PKU population.
Do you treat or manage adolescents with PKU who could be eligible for this study (please see who may or may not qualify below)? If so, you can help with recruitment by:
- Informing adolescents with PKU and their parents/guardians about the study; and
- Sharing the study link or QR code provided below with your adolescent with PKU and their parents/guardians.
Who may qualify:
- Adolescents (ages 12 to 17 years, inclusive) and their parent/guardian
- Adolescents diagnosed with PKU
- Adolescents and their parent/guardian who have access to internet and access to an electronic device (e.g., computer/laptop, tablet) to complete an online survey
- Adolescents currently managing their PKU with diet, medical food, or pharmacological therapy
- Adolescents and their parent/guardian who reside in the United States
Who may not qualify:
- Adolescents or their parent/guardian who have any limitation that might preclude them from participating in up to a 60-minute online survey
- Adolescents enrolled in Study to Evaluate the Safety and Efficacy of Pegvaliase in Adolescents (Ages 12-17) With Phenylketonuria (PEGASUS)
- Adolescent or their parent/guardian who reside outside the United States
This above information is intended for HCPs only. Please share the study link or QR code with interested patients.
Clinical Outcomes Solutions, a healthcare research company, is conducting this study on behalf of BioMarin Pharmaceutical Inc. For any questions about the study or the survey link and QR code above, feel free to contact Natasha Schumacher at COSstudy@clinoutsolutions.com or by phone at +1 (520) 325-9510.
Please share the study link or QR code with interested patients.
Click HERE to view PDF version of this information
______________________________________________________________________________________________________________
Posted May 17, 2023

PKU Study Recruiting Participants in the U.S
Do you have adolescent PKU patients who are eligible for this PKU Adolescent Pegvaliase study? If so, please help recruit by informing potential participants.
PEGASUS study (165-306): PEGvaliase: A Study of Use in adolescents
This clinical trial for adolescents with PKU is enrolling patients. Please see below qualifications:
Who may qualify:
- Adolescents aged 12–17 years old
(12 years of age at the time of consent)
- Diagnosed with PKU and unable to maintain blood Phe levels below 600 micromol/L their current prescribed treatment and prior sapropterin dihydrocholoride use
- Average blood phe concentration > 600 micromol/L over the last 12 months
- Participants must have an adult caretaker (age 18 and older) willing to observe the participant during pegvaliase injection and for a minimum of 1 hour following all pegvaliase injections during the study period
Who does not qualify:
- Adolescents previously treated with pegvaliase
- Adolescents who have used any medication intended to treat PKU within 14 days of the start of pegvaliase
- Adolescents who are unable to identify and/or communicate to others that they are experiencing symptoms of potential anaphylaxis (a severe allergic reaction to the medication), due to cognitive impairment or other reasons Please speak with your child’s PKU healthcare team to find out more information
Links to the study website and to CT.gov with more information on the study inclusion criteria, as well as contact information for sites are listed below
Study Website (link) - 165-306 Pegasus Study Website
Link to Clinicaltrials.Gov (link) - Clinicaltrials.gov
If you have questions, feel free to reach out to your BioMarin MSL or to Bridget Wardley (Bridget.wardley@bmrn.com)
_____________________________________________________________________________________________________________
Posted May 1, 2023

Jnana Therapeutics is developing a novel, orally administered medication for the potential treatment of PKU. SLC6A19 is the neutral amino acid transporter which is responsible for reabsorption of Phe from the proximal renal tubule and absorption of Phe in the small intestine. JNT-517 is an investigational oral small molecule inhibitor of SLC6A19 and is designed to block renal Phe reabsorption resulting in potentially increased urinary Phe excretion.
A Phase 1b clinical trial (JNT517-101) is being conducted of JNT-517 to assess the safety, tolerability, and effect on urine and blood levels of neutral amino acids (including Phe). The study is enrolling approximately 28 participants with PKU across 14 sites in the U.S. and Australia.
Click HERE to download flyer.
______________________________________________________________________________________________________________
Posted March 2023
Posted March 2023
Identification of linguistic markers revealing severity of symptoms in phenylketonuria:
A novel measurement tool for clinical trials
We are reaching out to inform you of a study being conducted by Susan Waisbren, PhD, a psychologist from Boston Children’s Hospital. The National PKU Alliance is funding this study, which is a collaboration among Boston Children’s Hospital, the PheFree Consortium, and scientists from IBM Watson Research, because it aligns with our mission of advancing research for the PKU Community.
Clinical trials in conditions affecting neurocognitive functioning usually require a single outcome measure that captures meaningful benefits of a novel therapy. In endeavors to demonstrate effects of new treatments in PKU, such a measure has eluded investigators. This is due to the heterogeneous and subtle deficits associated with PKU. Dr. Waisbren aims to evaluate a standardized instrument used to quantify parameters of verbal discourse as a means to identify linguistic markers revealing severity of symptoms in PKU. If successful, this study will provide the basis for further validation of a critical tool for demonstrating the benefit of novel treatments.
Dr. Waisbren will recruit 80 adults with PKU and 80 healthy adults without PKU (parents of children with PKU). All study activities, including a Zoom session, various questionnaires, and collection of a filter paper blood specimen (the latter in PKU patients only), will be conducted virtually.
If you are interested in this study for your adult patients with PKU or caregivers of your pediatric patients with PKU, please contact Dr. Susan Waisbren at Susan.Waisbren@childrens.harvard.edu or share the flyer with your patients.
______________________________________________________________________________________________________________
Posted October 2022
Patient-Centered Research Opportunity Aims to Personalize and Optimize Diet Treatment for Adults with Classical PKU
We are reaching out to inform you of a study being conducted by Dr. Shoji Yano, a clinical geneticist, and Kathryn Moseley, metabolic dietitian. The NPKUA is sponsoring the University of Southern California (USC) Keck School of Medicine in this research trial through our Research Grant Program because it meets the organization’s mission to improve the lives of adults with PKU.
This patient-partnered clinical trial is part of Dr. Yano and Kathryn Moseley’s ongoing research program with a broader goal to develop the long anticipated custom-tailored PKU diet treatment—to personalize and optimize the diet for adults.
The study aims to identify individual adults who may benefit clinically from addition of a large neutral amino acid (LNAA)-enriched medical food to their current diet plan. A body of evidence supports that additional dietary LNAAs block Phe uptake from blood to brain and improve neuropsychological function and quality of life in adult patients. Blood Phe concentrations, LNAA doses, and patient types most responsive to potential benefits of the dietary intervention are uncertain. This trial aims to clarify some of these unknowns.
All study procedures are done from participants’ homes. A placebo-control randomized clinical trial N-of-1 research design provides all participants with an individualized evidence-based evaluation of how well their current diet and blood levels are working for them, and whether the additional medical food may be more beneficial for them personally. Identifiable data will not be accessible to anyone until after the trial. At that time, only the participants, researchers, and the Institutional Review Board at USC will have access. Patients will be the first to receive their results when their study is completed, and they will choose whether they want to share the findings with their clinical teams.
The researchers plan to enroll 6 adults with classical PKU by December 15 with a January trial start date. Dietitians who are interested in the study for their adult patients with classical PKU, living in the U.S. with blood levels no higher than 900 µmol/L, are strongly encouraged to contact the Kathryn Moseley, M.S., R.D., Study Coordinator kmoseley@usc.edu.
______________________________________________________________________________________________________________
Posted October 2022
Please help recruit participants in MMA and PA natural history study
Real-world natural history data are needed to better understand methylmalonic and propionic acidemia as potential new treatments are being developed.
JUMP (Journey to Understand MMA and PA) is a natural history study being conducted with rare disease online research platform AllStripes to accelerate the understanding of MMA and PA for all stakeholders – families, academia, clinicians, and industry. JUMP is sponsored by HemoShear Therapeutics.
This short video from Kim Chapman explains the advantages of JUMP.

Patients Participate From Home
It takes about 15-20 minutes for patients and caregivers to sign up for a private account authorizing AllStripes to retrieve and process medical records on their behalf at no cost to the patient. Patients can access their records from their secure account. With consent, AllStripes uses de-identified patient data to generate disease insights.
Please Contact Your Families About JUMP
Here Dr. Chapman explains who can sign up for JUMP and how you can help to enroll participants.

Please contact your families with MMA and PA (up to 18 years of age) about joining JUMP.
You can download central IRB-approved flyers and a Q&A for you to share with families here: https://hemoshear.com/jump
______________________________________________________________________________________________________________
February 2022:

Links:
To learn more about the study or refer patients, please see clinicaltrials.gov (NCT04732429) to contact the nearest study site.
For families that want to learn more, the HERO website - mma-pahero.com - provides patient-friendly information For questions, please contact Chief Medical Officer Patrick Horn, MD, PhD at horn@hemoshear.com
______________________________________________________________________________________________________________

Arcturus is seeking participants ≥ 18 years of age with stable OTCD to take part in an ongoing clinical safety study of ARCT-810
Arcturus Therapeutics is developing a messenger RNA (mRNA) therapy for ornithine transcarbamylase deficiency (OTCD). Using mRNA is a new and fundamentally different approach to disease management than the use of traditional ammonia chelators and diet. The investigational ARCT-810 therapy contains mRNA with instructions to make fully functional OTC in the liver, thus addressing the root cause of OTCD with the potential to correct the urea cycle defect. mRNA technology uses lipid nanoparticles (LNPs) to protect the mRNA from degradation in the blood as it is distributed to the liver. The LNPs are designed to be taken up by hepatocytes and once inside the cell, they release the mRNA which can be translated to the OTC enzyme by the cells’ natural processes. Delivery of human OTC mRNA may help normalize ammonia and the other biochemical markers of ureagenesis.
This is an early Phase Ib single-dose study designed to assess the safety, tolerability and pharmacokinetics (metabolism and clearance) of ARCT-810. As such, participants who enroll must be adults with mild, stable OTC disease and good cognitive function. This may include women with only one OTCD gene (heterozygous). The information obtained from this study may allow the progression of ARCT-810 into studies of younger and sicker patients with OTCD. ARCT-810 is not a gene therapy, i.e., there is no alteration of the recipient’s genes and there are no viral components.
For more information about the Phase Ib clinical trial of ARCT-810 visit the study page on ClinicalTrials.gov: https://www.clinicaltrials.gov/ct2/show/NCT04442347?term=Arcturus&draw=2&rank=3
If you have questions or need help contacting one of the participating academic sites, please contact Manely Yafeh at manely@arcturusrx.com
David Geller, M.D. davidg@arcturusrx.com Vice President Pulmonary & Rare Diseases Arcturus Therapeutics
______________________________________________________________________________________________________________
Posted May 2020:
Updated list of clinical trials
Click here to return to Research Committee page